You’re reading the web edition of STAT’s Health Tech newsletter, our guide to how technology is transforming the life sciences. Sign up to get it delivered to your inbox every Tuesday and Thursday.
Are healthcare AI companies zooming in?
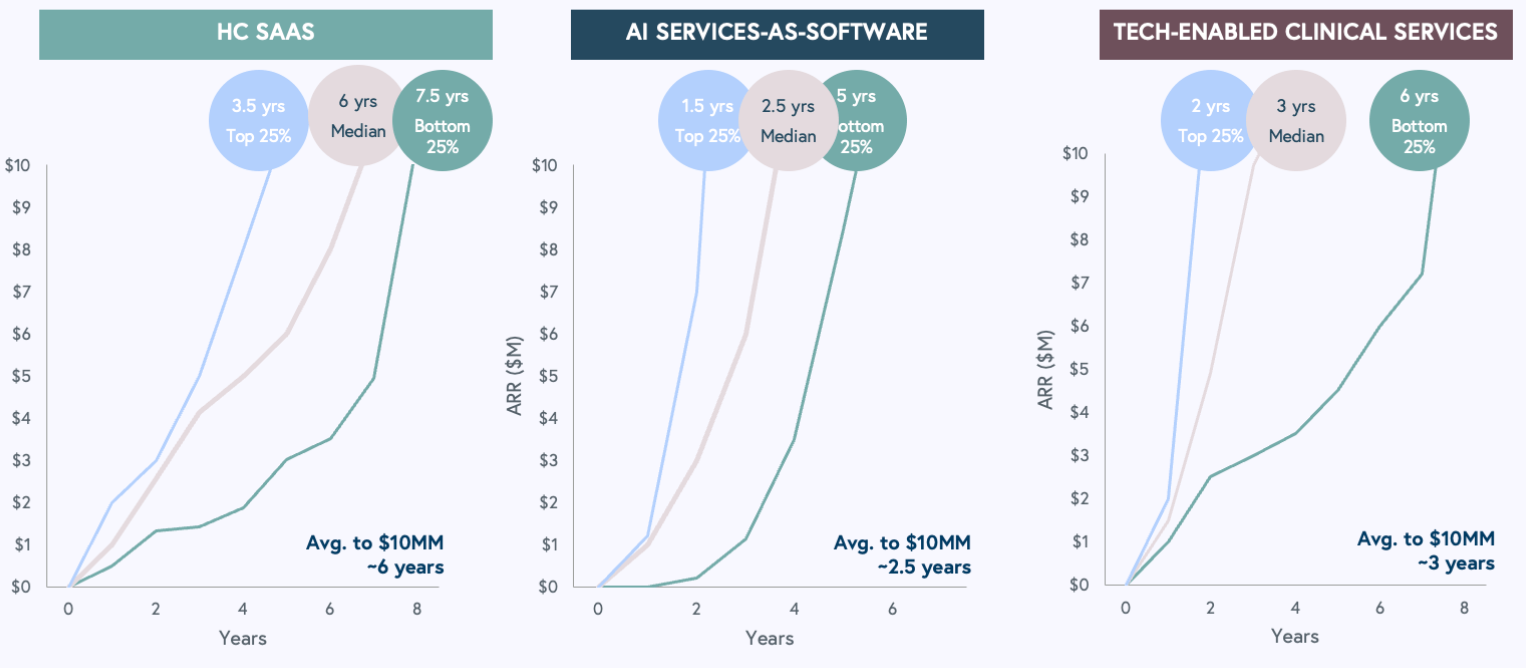
In a new report, Bessemer Venture Partners presents the first insights into the prospects of artificial intelligence software services companies which it sees as a new category in the broader health technology ecosystem. This category includes AI clinical writers, claims audit tools, and back-office automation technology. BVP has seen a rapid trajectory to $10 million in annual recurring revenue – a metric that investors consider a crucial indicator of a company’s health. It takes 2.5 years for AI companies to reach that $10 million threshold, compared to six years for healthcare software-as-a-service players and three years for tech clinical services companies. The data is based on a cohort of 20 AI companies.
BVP attributes the rapid start to rapid sales cycles driven by “buyers’ urgency to test and purchase AI-based solutions.” But! It should be noted that early revenues are often generated from trial programs that may not last long term. (BVP, of course, is not an independent party given that it invests in many of these companies.)
General Catalyst announces $750 million for health care in new fund
This morning, the venture capital giant General catalyst announcement 8 billion dollars in new capital, including 750 million dedicated to “health insurance”.
GC has recently made a number of aggressive bets in healthcare, including an offer to acquire an Ohio-based company Sum Santé as a testing ground for innovative ideas. Notable holding companies today include Commur, TransparentAnd Hippocratic. Before digital health became an established category, GC was a significant investor in a diabetes management company. Livongoincluding the sale for 18 billion dollars to Teladoc is one of the biggest health tech releases to date.
Why do Change Healthcare breach notifications take so long?
It’s October and some people have just received notification that their sensitive data has been compromised in the Changing healthcare ransomware attack that occurred in February. By law, people are supposed to be notified within 60 days if their data has been breached. So, what does that give?
As STAT’s Brittany Trang reportsit’s complicated. Partly, it’s unclear exactly when this 60-day clock is supposed to start. Additionally, Change is not a healthcare provider but a third party that processes insurance claims and helps facilitate insurance approvals and payments. In legal terms, they are a “business associate” of the companies that provide healthcare, and their responsibility is to inform their client – a doctor’s office or hospital – and not the person whose data have been compromised. You may notice that your breach notification arrived on Change Healthcare letterhead; this is because Change volunteered to take responsibility for the process.
Wednesday, Change parents UnitedHealth Group has confirmed that it has increased the number of people affected by the Change Healthcare cyberattack to 100 million. “We continue to notify potentially affected individuals as quickly as possible, on an ongoing basis, given the volume and complexity of the data involved and the investigation is still in its final stages,” a spokesperson reiterated to STAT.
Granted, that’s a lot of people to warn, but the mess of breach notifications underscores what many observers see as the sad state of healthcare cybersecurity. In fact, last month, Sens. Ron Wyden (D-Ore.) and Mark Warner (D-Va.) introduced a bill that would impose stricter cybersecurity rules on health care organizations and remove the cap on the amount of fines violators can be assessed. UnitedHealth Group made $22 billion in profit last year. The idea is that if you want to change cybersecurity practices, you have to hit them where it hurts. Read more here
Retinal implant shows promise for people with severe vision loss
 Scientific Society
Scientific Society
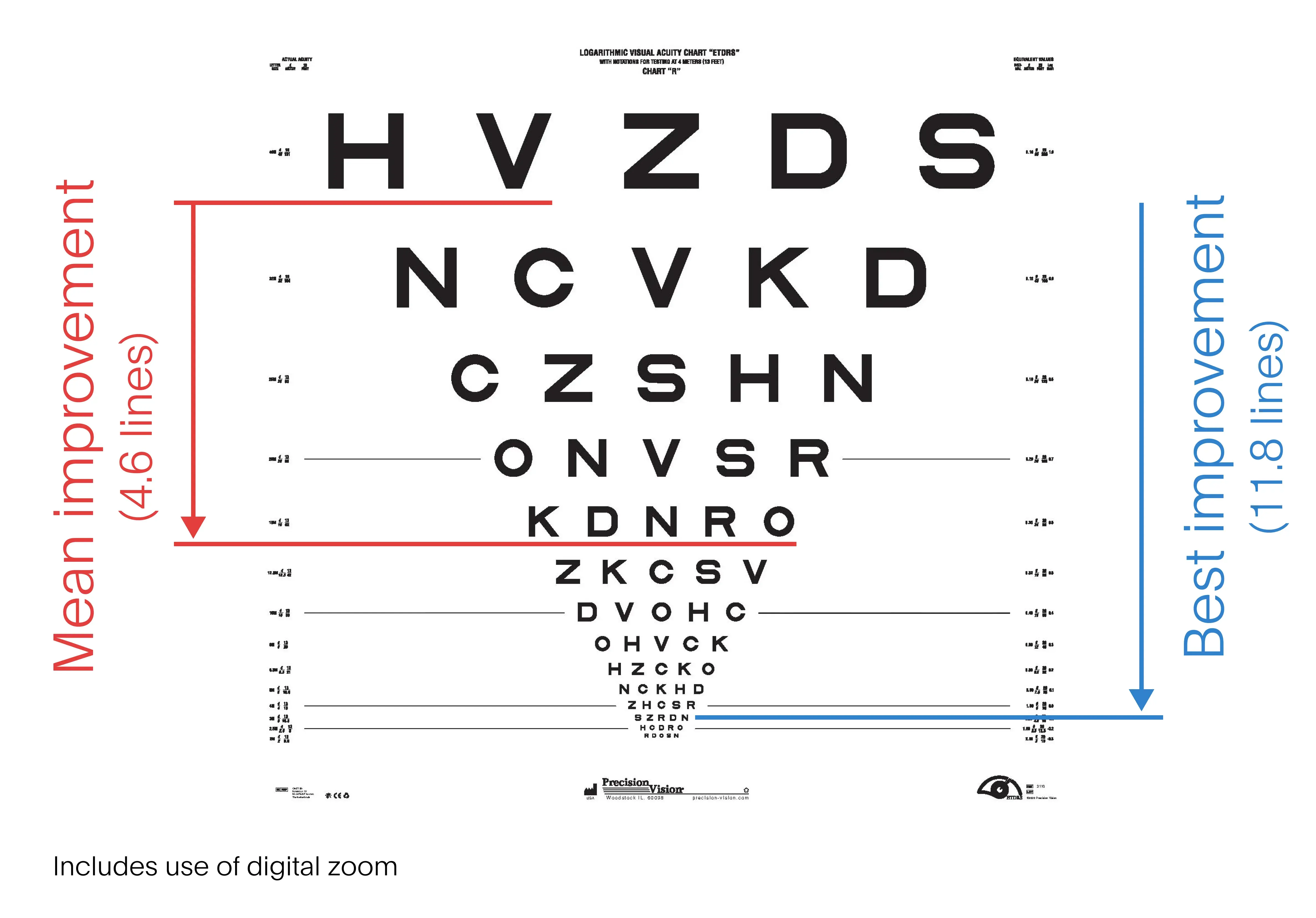
Preliminary clinical trial data shows that a retinal implant restored vision in people whose central visual field had holes or blurred spots. Scientific Society reported this week. Trial participants could read text and recognize playing cards when using the implant, even if they were legally blind. The findings, which have not been published in a peer-reviewed publication, hold promise for millions of people suffering from age-related macular degeneration, a leading cause of vision loss.
Called the Prima System, the implant relies on a camera mounted on glasses and a handheld computer. The camera captures infrared light and transmits it to the implant which stimulates the retina.
Science Corporation enrolled 38 people in the study, six of whom dropped out before testing. As STAT’s Timmy Broderick reportsAfter twelve months of use, participants could read, on average, nearly five additional lines, or 23 letters, on an eye chart. This equates to an improvement in a person’s vision from 20/320 to 20/200, which is the threshold for blindness in the United States. Read more here
Walmart’s Pharmacy Delivery Campaign and Hiring a Big Company
There has been a lot of noise at HLTH this week but two developments caught my attention:
- Walmart announcement that it will offer same-day pharmacy delivery in 49 states by the end of January. The offer is already available in six states. Walmart news follows AmazonIt is recent expansion Same day pharmacy delivery.
- Early-stage venture capital firm Define businesses added old Human CEO Bruce Broussard as a partner. Big shot!
WADA Panel Approves Long-Sought Remote Proctoring Update
THE American Medical AssociationIt is CPT Editorial Committeeresponsible for developing the billing codes used by doctors, announced that it had accepted proposed updates to remote monitoring codes that will take effect in 2026. Although exact details have not been released, the update will allow doctors to bill for providing devices in cases where fewer than 16 days of data are collected as well as billing for less than 20 minutes of management services linked to monitoring. The effort to update the codes took several years, with some abandoned efforts along the way.
Notes from the new device chief of the FDA and other agencies
This week, the Food and Drug Administration confirmed which many people probably already suspected: Michelle Tarver will be the new director of Center for Devices and Radiological Healthwhich oversees medical devices and hot topics such as artificial intelligence tools and laboratory-developed tests. Tarver joined the agency in 2009 and has served as the center’s interim chief since Jeff Shuren announced his departure this summer.
- The FDA too released new information about the upcoming inaugural meeting of the Digital Health Advisory Committee November 20 and 21. During the public meeting, which will be streamed live on YouTube, the new committee will discuss “Total Product Lifecycle Considerations for Medical Devices Based on Generative Artificial Intelligence.” Comments for the meeting schedule must be submitted by November 1.
- Speaking at a conference for the biosimilar and generic drug industry, FDA chief Robert Calif suggested that the agency would use artificial intelligence tools to detect “cheating” in clinical trials, Bloomberg Law Reports.
What we read
- What’s on the agenda for after the congressional election: Chinese biotechnology, Medicare payments, ACA subsidies, STATUS
- Artificial intelligence-assisted colonoscopy for polyp detection: systematic review and meta-analysis, Annals of internal medicine
- Performance of multimodal artificial intelligence chatbots evaluated on oncology clinical cases, Open JAMA Network